-

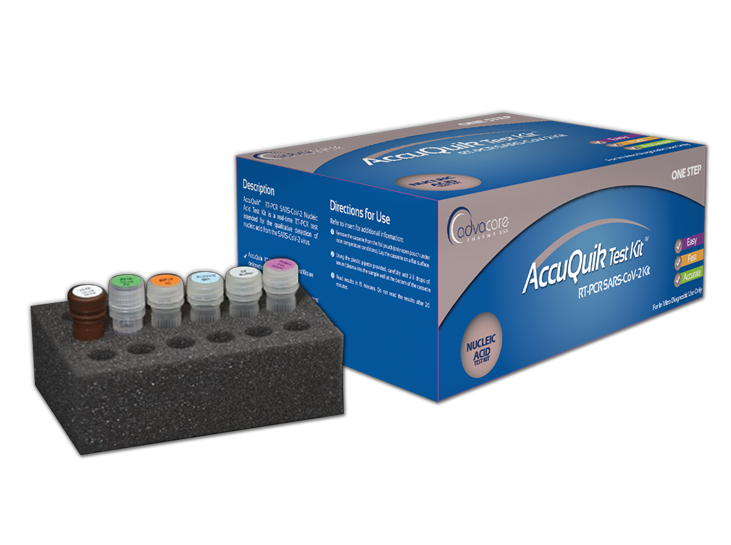
AccuQuik™ Test Kits
Diagnostic Test Kits Manufacturer
-

AccuQuik™ Test Kits
Diagnostic Test Kits Manufacturer
-
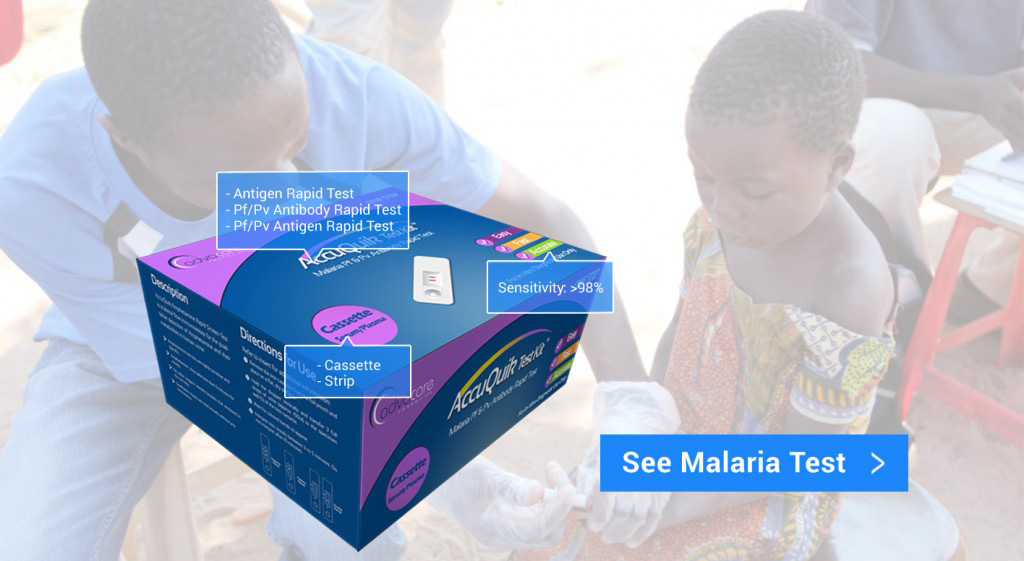
AccuQuik™ Test Kits
Diagnostic Test Kits Manufacturer
Why AccuQuik™?
As an American brand, AccuQuik™ places the highest priority on the quality of our products.
As we look to serve the needs of the developing world, our diagnostic test kit products are an affordable solution to detecting and treating infectious diseases, pregnancy, and narcotic usage.